, that is a longer period
of functioning in time. So, for example, if the time of functioning of a Mu- meson equals
only 2ž10-6 sec. (two millionth parts of a second), then the time of existence
of neutrons and protons is much longer.
Nowadays more than 200 appellations of
fng. units, circulating in sublevels A - B, are known.

Level C
More than one hundred atomic elements of the periodical
system of Mendeleev constitute systemic formations of the sublevel C.
The functional features of these units have been studied more deeply than the
characteristics of the units of sublevels A - B. Their inner
structure by now is also very well-known.
The structural difference between them comes down to the number
of protons, neutrons, mesons and electrons, entering them, but every next addition of a
couple proton-electron to a system abruptly changes the functional characteristics of the
whole combined unit entirely and this serves as an obvious confirmation of the regulation
of the number of fnl. cells in every given system.
The field of spatial spreading of units of the level
C is (as well as for units of sub levels A - B) the whole
field of the Universe visible by us.
The principal mass of any unit of the present level - atom - more
than for 99,9% is concentrated in its nucleus, the dimensions of which is 10-13
cm, that is 105 times less the dimensions of the atom itself (10-8
cm). So, if to imagine the dimensions of an atom in the form of a football field (with the
diameter 100 m), then the atomic nucleus would correspond to a pellet with the diameter
only 1 mm. Nuclei have complicated structure of fnl. cells. The principal elements filling
them in as fng. units are the nuclear particles of the sublevel B - nucleons:
protons and neutrons. Their masses of rest are equal accordingly to 1,00812 and 1,00893
of ideal units. The mass of electrons forming part of any atom is almost 2000 times less
(5.5ž10-6 i.u.) the mass of nucleons. The particles intermediate by mass
between electrons and protons and forming part of nucleus - Mu- and Pi- mesons - have
bigger masses than electron in 210 and 275 times accordingly.
The formation of stable and compact atomic nucleus from
nucleons - protons and neutrons - can be explained by the arising of nuclear power,
nuclear links between them, and mesons are responsible for that. Nucleons are exchanging
between themselves with mesons turning in turn into now proton, now neutron, while a
proton can form links with a limited number of neutrons, and vice versa, a neutron gets
links with a definite number of protons. Therefore the stability of nuclei depends on
a number of protons and neutrons that are filling in the fnl. cells of a structure of
a nucleus.
The number of protons defines the magnitude of the positive
charge of a nucleus, and that is the most important characteristic of an atom, as the
number of electrons in an electroneutral atom and finally functional features of every
atom depend on it.
The mass of a nucleus ('the mass number of an atom' - A),
being a sum of masses of all protons and neutrons forming part of a nucleus, is
practically equal to the mass of the whole atom.
Nuclei, having the same number of protons, can have a different
number of neutrons, that is to be isotopes. Almost all chemical elements have several
isotopes. The elements, having charge of the nucleus from 40 to 56, that are located in
the middle of the periodical system, have the most numerous isotopes (per 6-10 each).
The number of lasting (stable) isotopes is considerably less than the number of unstable,
that is radio-active ones. The stability of nuclei depends on the number of protons and
neutrons, forming them as fng. units, and on their ratio. In structures of fnl. cells of
maximum stable nuclei of light elements there is one neutron per each proton. With the
growth of the charge of the nucleus the increase of the number of neutronic fnl. cells
outstrips the increase of the number of protonic ones. In nuclei with A < 25
every nucleon is being dragged up by nuclear forces to all the rest nucleons, in nuclei
with á = 25 - 30 the nuclear forces begin to be sated (that is every nucleon
is being dragged up not by all the rest nucleons, but only by those that closely surround
it). In nuclei with á > 50 the force of electrical repulsion between
protons more and more noticeably counteracts to forces of nuclear link. Any two protons,
being located in diametrically opposite sides of a big nucleus, continue to interact
electrically while for nuclear interaction they are located already too far one from
another. On the contrary, in the lightest nuclei all nucleons are located so near one
from another that the effect of the force of repulsion is fully neutralised by nuclear
attraction. It is natural that the force of repulsion as a functional characteristic
of the present structure is striving to destroy large atomic nuclei contrary to the
restraining influence of the functional characteristic of nuclear attraction, and
therefore the magnitude of forces of the connection of such a nucleus would depend
on a ratio between these two forces. This balance of some very heavy nuclei is quite
unsteady; such nuclei become unstable and strive to a spontaneous desintegration, that
is are radio-active. This happens mainly when there is shortage or excess of neutrons
in a nucleus. Depending on the kind of particles emitted by a nucleus one can distinguish
several types of radio-active desintegration: protonic, positronic, electronic, etc.
Massive positively charged nuclei of atoms create around
themselves a powerful electromagnetic field, in which in fnl. cells of atomic orbitals
in a definite way electrons are placed. The number of electrons in an atom (equal to the
charge of its nucleus) as well as their location in space, determine all chemical, and
consequently, functional features of each element. Therefore any change of the fnl.
characteristics of any substance as well as the transformation of some substances into
others are linked with the change of internal structure of fnl. cells of their atoms,
with number and composition of filling them in fng. units of lower sublevels.
The planetary model of composition of an atom, which existed
until recent time, could not explain not only all the variety of functional (chemical)
characteristics of different atoms, but even the thin structure of spectrums of radiation.
Therefore nowadays the model of atom gains a firm hold more and more, which consists of a
nucleus, enveloped by closed stagnant waves of electrons, forming 'an electronic cloud',
in which the movement of electrons along definite trajectories is impossible to imagine,
as for example the movement of planets around a star. Hence there is always uncertainty
in the position of electrons, in determination of their location.
The dual nature (dualism) of electron, having characteristics
of both a particle and a wave, leads to the fact that its movement cannot be described
by a definite trajectory. A trajectory is being 'washed away', a strip of uncertainty
appears, within the bounds of which the electron is located. At any moment of time it
is impossible to define both the position in space and the velocity (or impulse) of the
electron. The movement of the electron is described with the help of a wave function,
being a function of spatial coordinates. The wave function should be synonymous, final
and continuous in space. It is equal to zero in places where the electron cannot be
located. As a result of the calculation of a wave function we get volumetric figures -
'electronic clouds', that have the name of atomic orbitals. They are described by three
constant whole numbers - quantum numbers. Their meanings indicate the probable location
of an electron in an atom.
The 'main quantum number' determines the most probable distance
of an electron from the nucleus of an atom, that is an average radius of electronic layer
(orbit). The 'azimuth quantum number' determines the moment of quantity of movement of an
electron and characterises electronic sublayers (sublevels of energy), forming every layer.
The 'magnetic quantum number' determines the orientation of every sublayer in space that
cannot be arbitrary.
So then, electrons in every atom are located in layers, layers
are divided into sublayers, every sublayer consists of oriented in space fields - atomic
orbitals, in the fnl. cells of which the probability of being of electron is the topmost.
The position of an electron in an atom depends also on its own moment of quantity of
movement, which is appearing as if because of 'rotation' of the electron around its axis.
At the same time, an electron, having some electrical charge, reveals its own magnetic
moment, characterised with the spin quantum number. Due to the fact that the rotation
of an electron can be going in two mutually opposite directions, maximum two fng. units -
electrons can fill in the couple of fnl. cells of each atomic orbital, moreover both of
them should have opposite (antiparallel) spins.
Since the whole energy of an electron is its principal
characteristic, which is taken into account by the wave equation, its magnitude defines
the probability of being of an electron in a fnl. cell of this or that atomic orbital.
The levels of energy of an electron cannot be arbitrary as they should be a multiple of
Planck's constant. It is known that during transition from an upper allowed level to
a lower one (closer to the nucleus), an electron frees itself from a surplus of energy
emitting it in the form of electromagnetic waves. In the case of absorption by an
electron of energy an opposite process is going on - the atom is being excited. In
an unexcited atom the electrons have minimum energy and consequently are situated in
fnl. cells of atomic orbital, that are located 'closer' to the nucleus. Precisely
speaking, the electron occupies the functional cell of that atomic orbital, the staying
in which allows it for the most part to be situated near the nucleus of the atom.
It is natural to suppose that electrons participating in the
formation of an electronic cover of an atom, are composing themselves first of all in
fnl. cells of atomic orbitals, characterised by the smallest energies, and after filling
them in, on more and more upper levels, that is the order of formation of electronic
cover of an atom, the order of its development together with the growth of charge of
the nucleus and corresponding increase of the number of electrons coincides with the
sequence of location of atomic orbitals according their energies.
We have stopped on the description of structures
of systemic formations of the level C in detail for several reasons.
Firstly, on the basis of additional knowledge obtained by
scientists during recent years as a result of experiments at powerful accelerators
of particles, our ideas about the construction of the atom are undergoing bigger and
bigger changes, and the model of its structure becomes more and more complicated.
Secondly, the knowledge of the construction of atoms is
essential in order to understand the genuine picture of the formation of the material
Universe, because this organisational sublevel nowadays is primary, since in its
construction the peculiarities of the evolution of lower for us sublevels of Matter
are revealed, its variations define functional interactions of material structures
of higher levels.
Thirdly, the fine structure of the construction of the atom
and its components should demonstrate that material units are not spontaneously developed
formations. All of them, even at a so relatively low organisational level, represent
systemic formations of Matter created in accordance with the strictly definite laws from
functioning units of lower sublevels, bearing corresponding functional load, the character
of which would be more clear on considering the construction of systems of the next levels
in the general line of the organisational evolution of the material substance.
Thus, the complex elements of the sublevel C - atoms
- according to their construction allow one to set out in the order of growth of the
charges of their nuclei. Precisely this was actualised by D.I. Mendeleev in 1869 and
as a result of that a rather methodical periodical system of elements appeared bearing
his name. Since the charge of a nucleus defines the number of electrons, then atoms of
every following element have one electron more, than the atoms of the previous one.
The most widespread element of the Universe is hydrogen. About
half of the mass of the Sun and most of other stars falls on its part. Gas nebulas,
interstellar gas contain it. It is forming stars. In depths of stars the transformation
of the nuclei of atoms of hydrogen into the nuclei of atoms of helium is going on while
elements of sublevels A and AA are being radiated, afterwards filling fnl.
cells in different systemic formations of the Universe.
There is no cause to turn down a supposition that the motion
of Matter in quality (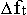 ) during the definite historical period (-t) was going
on in the Universe exactly along the lines of construction of the structural formations
of atoms (that is along the sublevel C) from the simplest elements - hydrogen,
helium - to the more and more complicated. How long this period was lasting
(
) during the definite historical period (-t) was going
on in the Universe exactly along the lines of construction of the structural formations
of atoms (that is along the sublevel C) from the simplest elements - hydrogen,
helium - to the more and more complicated. How long this period was lasting
(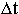 )
and how far newly formed elements have spread in space
(
)
and how far newly formed elements have spread in space
(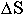 ),
it is impossible to say precisely for the time being, but right now it is possible
to make a few certain deductions.
),
it is impossible to say precisely for the time being, but right now it is possible
to make a few certain deductions.
Firstly, the process of the formation of elements of the level
C - atoms - was going on with the absorption of considerable quantities of kinetic
energy, its systemic binding in structures of units of the new level, transferring it into
hypothetical power potential. Bearing in mind that the total quantity of energy for the
whole aggregate Matter is a constant magnitude, during the increase of the number of
heterofunctional atomic elements and the further integration of their structures, the
item of the kinetic energy was decreasing more and more, which resulted in the appearance
in the Universe of peculiar condensations of material formations - stars, alternating with
relatively boundless spaces practically free of energy. In other words, as a result of the
integrative process of systemic organisation within the limits of the level C during
the above stated phase of the Evolution of Matter, the energy along the whole length of
space-time of the Universe was grouped into relative concentrations - galaxies and spots -
stars, although the dimensions of those concentrations and spots, expressed in the metric
system, have rather impressive magnitudes.
Secondly, by the same reason leading to the lowering of the
numerator in the formula 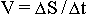 the velocity of the spreading of every material formation of subsequent
organisational levels is also decreasing at the end striving to zero.
the velocity of the spreading of every material formation of subsequent
organisational levels is also decreasing at the end striving to zero.
Thirdly, in the process of the motion of Matter in quality
along the level C, started, as we have already said, from the formation of hydrogen
and helium, more than 100 types of structures of different elements were assembled. The
appearance of more cumbersome atoms than uranium and plutonium is made difficult owing
to the exceeding of forces of repulsion of protons in their nuclei over forces of nuclear
link. As a result in such atoms a desintegration to elements with more steady nuclear
structures is taking place. Because of this any further motion of Matter in quality
along the level C became impossible and it got over to the level D, to the
examination of which we shall pass herein after. However, before that we shall make some
remarks that are very important for our study.
All the particles of sublevels A, AA, AB,
B and C, examined by us, form a group of functioning units, which serves
as a foundation for the evolution of all further systemic formations of Matter. The total
number of said elements exceeds 300, however, each combination constitutes a new variant
of the systemic organisation on the given level and leads to a creation of a new
functioning unit with strictly definite characteristics. Without knowledge of the
regularities of the formation of these units and the distinguishing peculiarities of the
alteration of their functional features it is impossible to cognize the whole picture of
the Evolution of Matter. We also should remember that for all units and systemic formations
of levels A - C the laws of the general theory of systems are typical
and valid continuously, in accordance with which every functional cell of any systemic
formation should be occupied and always is occupied only by the functioning unit
corresponding to it. Therefore in any nucleus the fnl. place of proton should be occupied
only by a proton with the strictly corresponding fnl. characteristic, but not by a hyperon
or meson. All fnl. cells of atomic orbitals are being filled in by electrons with strictly
specific characteristics, and in the case of alteration of one of them the electron cannot
stay already in the given fnl. cell, which entails a change of fnl. features of the whole
system of the given atom. At the same time all chemical compounds of substances are based
on the temporal diversity of the periods of existence of fnl. cells of atomic orbitals
and fng. units - electrons.
The dual nature of functional cells and functioning units is
confirmed by the famous theory of Dirak about antiparticles. As it is known, its idea
comes to the following. If all positions with negative energy (fnl. cells) in any systemic
formations are already occupied by fng. units - electrons, no one new electron can get over
to these positions from positions with positive energy, since, as we know, each fnl. cell
can be occupied only by one fng. unit - there is no place there for another one. However,
if by some reason an electron with negative energy leaves its fnl. cell, among positions
with negative energy one position will remain not filled in, or, as one used to say, a
'hole' will appear. But lack of negative charge is perceiving as a positive charge and
lack of negative energy - as ordinary positive energy: minus by minus gives plus. Dirak's
theory predicted the possibility of the appearance of positively charged electrons,
which later got the name positrons. If an ordinary electron with negative charge meets
a positron, it can fill up the hole, that is 'fall' to a vacant place among positions
with negative energy. The surplus of energy would be transmitted to the electromagnetic
field and the background of electrons with negative energy would become uniform everywhere,
that is not being observed. So if all the positions with negative energy are occupied,
that is the normal and main condition of the background as a whole: then there are no
holes-positrons. Interaction of an electron (fng. unit) with a positron (fnl. cell)
results in the annihilation of their particular qualitative features while they themselves
become a part of a structure of a higher systemic organisation.
The principle of duality of fnl. cells and fng. units is
attributed also to structures of bigger elements. Thus in an experimental way, as it is
known, antinuclei of isotope of helium-3 were detected. It is not excluded, that one of
the continuations of this theory is logically connected with a solution of the mysteries
of large black holes in outer space and the possibility of existence of the antiworld.
But this is the subject of another study.
Considering the nature of interactions of different elements
of sublevels A - C we can subdivide them in accordance with universally
recognised classification into four types, differing one from the other: strong,
electromagnetic, weak and gravitational. Their big difference is seen from the
comparison of the relative intensities of interactions, which relate correspondingly
as 1:10-2:10-5:10-38. The gravitational interaction
determines the structure of outer space, electromagnetic - the structure of atom and
molecule, the strong interaction defines the structure of nucleus. All particles of
mentioned sublevels are exposed to the weak interaction with the exception of photon.
Moreover it is necessary to keep in mind, that certain symmetries are attributed to all
said interactions. And if for some interactions they are closely connected with the
symmetry of space-time, then for the others they submit to the laws of internal symmetry
of interactions.
Before the continuation of our study along the coordinate
of quality we should stop at one more important moment. As we have already noted,
parallel with the motion of Matter in the sublevel C, that is a functional
differentiation of its cells and units, simultaneous concentration of elements into
star bodies, which spatial volume was incomparably less than left materially rarefied
interstellar space, was taking place. As a result, with the help of already mentioned
formula of the whole energy of a system of spots
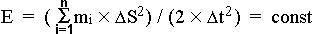
one can make a range of interesting conclusions.
It is known, that because they are bound in star structures,
displacements of material formations of the sublevel C are extremely decreased
(that is 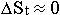 , and
, and
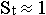 ), while energy of
the whole system remains as a constant magnitude. Then the formula of the whole energy
for systemic elements, having concentrated in space, will be transformed into the sense
expression
), while energy of
the whole system remains as a constant magnitude. Then the formula of the whole energy
for systemic elements, having concentrated in space, will be transformed into the sense
expression  . But
if to take into consideration, that a total mass
. But
if to take into consideration, that a total mass  is an object of functional differentiation
is an object of functional differentiation
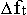 , then the said
dependence one can write as
, then the said
dependence one can write as 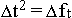 , which means, that in conditions of the limitation of movement in space,
characteristic for material particles concentrated in star-planetary bodies of the
Universe, for keeping the trend of the tensor of the Evolution of Matter the motion
in quality (
, which means, that in conditions of the limitation of movement in space,
characteristic for material particles concentrated in star-planetary bodies of the
Universe, for keeping the trend of the tensor of the Evolution of Matter the motion
in quality (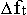 ) should be in quadratic dependence from the motion of Matter in
time. Owing to this the increment of functional features of material systems,
concentrated in star-planetary formations, for some region of the Universe is passing
considerably faster, than if it was happening for the whole material substance uniformly
stretching and moving along the space of the Universe.
) should be in quadratic dependence from the motion of Matter in
time. Owing to this the increment of functional features of material systems,
concentrated in star-planetary formations, for some region of the Universe is passing
considerably faster, than if it was happening for the whole material substance uniformly
stretching and moving along the space of the Universe.
From the same equations it follows that for material
systems - fng. units the movement of which in space is practically limited
(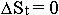 ),
the time of functioning is equal to the square root from their functional total mass
),
the time of functioning is equal to the square root from their functional total mass
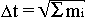 , that is the
less is their total mass the shorter is the period of their functioning and,
correspondingly, existence. Figuratively speaking, the obtained equation one
can name "the formula of death of all frozen".
, that is the
less is their total mass the shorter is the period of their functioning and,
correspondingly, existence. Figuratively speaking, the obtained equation one
can name "the formula of death of all frozen".
It is appropriate to note here, that with every subsequent
organisational level of the Evolution of Matter the fng. units are bearing more and
more fnl. loads, that is the coefficient of their polyfunctionality is increasing.
And the more complex in organisation a structural level of Matter is, the higher
this coefficient will be. The noted factor facilitates the solution of the problem
of acceleration of the motion of Matter in quality
(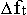 )
in conditions of the limited space
(
)
in conditions of the limited space
(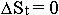 )
of star-planetary formations.
)
of star-planetary formations.

[ To Contents ]
[ Next ]
![]()
![]()
![]()
![]()